
Andy Napper
Title: Professor of Chemistry
Subject Area: Chemistry
Office Location: MAS323
Phone: (740) 351-3100
Email: anapper@shawnee.edu
Education
B.Sc., University College of Wales, Swansea, U.K
Ph.D., University of Pittsburgh
About
Dr. Napper is a physical chemist who teaches both freshman level chemistry courses (CHEM1121/1141/1142), analytical chemistry (CHEM3323/3325), and the senior level physical chemistry sequence (CHEM4431/4432).
Prof. Napper's research has spanned the fields of computational chemistry, laser spectroscopy, and fermentation science. He served as department chair for Natural Sciences from 2012 – 2018, with a brief appointment as acting dean of arts & sciences during the 2015 – 2016 academic year. He has held several positions with the Central Ohio American Chemical Society, including chair and secretary.
In his down-time, Dr. Napper enjoys reading, star-trek, and brewing the occasional batch of beer.
Undergraduate Research Projects
1. Construction of CdSe Quantum Dot Photovoltaic Cells
CdSe quantum dots of various sizes were prepared using a previously published procedure. These quantum dots were physisorbed onto a nanocrystalline rutile TiO₂ layer and a photovoltaic cell was constructed using two layers of ITO (Indium Tin Oxide) doped glass, a graphite electrode, and I₂/I₃⁻ electrolyte. iV (current-voltage) curves were measured and used to calculate efficiency.
Alkane thiols of various lengths, ω terminated with the COOH functional group, were used to covalently attach the quantum dots to the TiO₂ layer. Efficiencies were compared with the physisorbed system.

Photovoltaic cell construction

Vials Containing Synthesized CdSe Quantum Dots

Fluorescent Properties of CdSe Quantum Dots
2. Analysis of Banknotes for Cocaine Using Single-Ion-Mode (SIM) Gas-Chromatograph/Mass-Spectrometry (GC/MS)
Cocaine was extracted from $5, $10, and $20 bills using dilute HCl, followed by treatment with dilute NH₃. The cocaine was concentrated using a C-18 solid phase extraction column, followed by elution with methanol. Injection of a 1 µL sample into a GC-MS allowed for identification of cocaine. After a standard curve was constructed, it was possible to determine how much cocaine was present on each bank note. Amounts ranging from nanograms (10⁻⁹ g) to micrograms (10⁻⁶ g) were detected on each note.
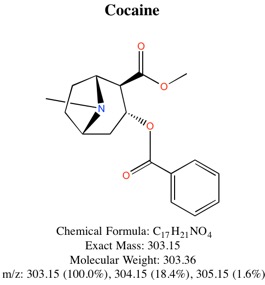
Chemical Structure of Cocaine
3. Construction of a Diode-Laser Light-Scattering Spectrometer and the Determination of Kinetic Parameters of Colloidal Sulfur Formation
A diode-laser light scattering spectrometer was constructed using a previously published procedure. Colloidal sulfur was generated using the reaction between dilute HCl and Na₂S₂O₃, and the kinetics of sulfur formation was generated by measuring scattered laser light intensity vs. time.

Experimental Set-Up (Side-View)

Experimental Set-Up (Top-View)
4. High-Resolution Infrared Spectroscopic Study of Polyatomic Gases
HCl(g), HBr(g), HI(g), and C₂H₂(g), along with the isotopomers DCl, DBr, DI, and C₂D₂ were generated and collected in an IR gas cell. High resolution (0.5 cm⁻¹) FTIR spectra were collected, and rotational-vibrational (ro-vib) parameters were calculated by analysis of the P-(Q)-R branches of the ro-vib spectra.
5. Fusel Alcohol Concentration as a Function of Fermentation Temperature Monitored Using GC/MS
Fermentation of sugars at elevated temperatures leads to generation of undesirable fusel alcohols. These fusel alcohols contribute to an off-taste of distilled spirits, as well as contributing to hang-overs. This project investigated the fermentation of glucose using brewers yeast (Saccharomyces cerevisiae) at various temperatures and fermentation times. Concentrations of the fusel alcohols: 1-propanol, 1-butanol, and 1-pentanol were determined by injection of 1 µL samples into a GC/MS, and analyzed using an internal standard.
Service
Select service work:
- Chairperson, Department of Natural Sciences: May 2012 – May 2018
- Acting Dean, College of Arts & Sciences: September 2015 – June 2016
- Science fair judge, 2001 – present
- AQIP category 2 team member, 2016 – 2017
- Academic Appeals Committee, 2012 – 2018
- Academic Affairs Strategic Plan Committee, 2016
- Safety & Security Committee, 2014 – 2016
- Continuous Improvement & Mission Committee, 2013 – 2015
- University Technology Advisory Committee, 2004 – 2011
- Co-chair, Ohio Department of Higher Education, Transfer Pathways in Math & Science, 2017 – 2018
- Chair elect (2013), Chair (2014), Past-Chair (2015), Secretary (2016 – 2017), Central Ohio Valley American Chemical Society
Publications
- N. Balabai, B. Linton, A. Napper, S. Priyadarshy, A. P. Sukharevsky, and D. H. Waldeck; “Orientational Dynamics of β-Cyclodextrin Inclusion Complexes,” The Journal of Physical Chemistry B; 1998; 102(48); 9617 – 9624. DOI: 10.1021/jp982756e
- I. Read, A. Napper, R. Kaplan, M. B. Zimmt, and D. H. Waldeck; “Solvent-Mediated Electronic Coupling: The Role of Solvent Placement,” Journal of the American Chemical Society; 1999; 121(47); 10976 – 10986. DOI: 10.1021/ja992281k
- I. Read, A. Napper, M. B. Zimmt, and D. H. Waldeck; “Electron Transfer in Aromatic Solvents: The Importance of Quadrupolar Interactions,” The Journal of Physical Chemistry A; 2000; 104(41); 9385 – 9394. DOI: 10.1021/jp001727c
- A. M. Napper, I. Read, and D. H. Waldeck, Nicholas J. Head, Anna M. Oliver, and M. N. Paddon-Row; “An Unequivocal Demonstration of the Importance of Nonbonded Contacts in the Electronic Coupling between Electron Donor and Acceptor Units of Donor-Bridge-Acceptor Molecules,” Journal of the American Chemical Society; 2000; 122(21); 5220 – 5221. DOI: 10.1021/ja000611r
- R. W. Kaplan, A. M. Napper, D. H. Waldeck, and M. B. Zimmt; “Solvent Mediated Coupling Across 1 nm: Not a π Bond in Sight,” Journal of the American Chemical Society; 2000; 122(48); 12039 – 12040. DOI: 10.1021/ja002264r
- A. M. Napper, H. Liu, and D. H. Waldeck; “The Nature of Electronic Coupling through Insulating Barriers on Au Electrodes. The Importance of Chain Composition, Interchain Coupling, and Quantum Interference,” The Journal of Physical Chemistry B.; 2001; 105(32); 7699 – 7707. DOI: 10.1021/jp0105140
- R. Kaplan, A. M. Napper, D. H. Waldeck, and M. B. Zimmt; “The Role Played by Orbital Energetics in Solvent Mediated Electronic Coupling,” The Journal of Physical Chemistry A.; 2002; 106(10); 1917 – 1925. DOI: 10.1021/jp011603f
- A. M. Napper, I. Read, D. H. Waldeck, R. W. Kaplan, and M. B. Zimmt; “Electron Transfer Reactions of C-shaped Molecules in Alkylated Aromatic Solvents: Evidence that the Effective Electronic Coupling Magnitude Is Temperature-Dependent,” The Journal of Physical Chemistry A.; 2002; 106(18); 4784 – 4793. DOI: 10.1021/jp0204455
- A. M. Napper, I. Read, R. Kaplan; M. B. Zimmt, and D. H. Waldeck; “Solvent Mediated Superexchange in a C-Clamp Shaped Donor-Bridge-Acceptor Molecule: The Correlation between Solvent Electron Affinity and Electronic Coupling,” The Journal of Physical Chemistry A.; 2002; 106(21); 5288 – 5296. DOI: 10.1021/jp014529+
- A. M. Napper, N. J. Head, A. M. Oliver, M. J. Shephard, M. N. Paddon-Row, I. Read, and D. H. Waldeck; “Use of U-shaped Donor-Bridge-Acceptor Molecules To Study Electron Tunneling through Nonbonded Contacts,” Journal of the American Chemical Society; 2002;124(34); 10171 – 10181. DOI: 10.1021/ja025683s
- A. M. Napper, Haiying Liu, H. Yamamoto, D. Khoshtariya, and D. H. Waldeck; “Effect of Molecular Properties on Electron Transmission through Organic Monolayer films” in “Molecules as components of electronic devices”; Marya Lieberman, editor; ACS Symposium Series 844; American Chemical Society: New York, NY; 2003, 62 – 75. DOI: 10.1021/bk-2003-0844.ch006
